Research on the Expansion Forces of Metal Hydrides During Hydrogen Absorption and Their Influencing Factors
The storage of hydrogen in metal hydrides offers an efficient, loss-free, and safe form of both stationary and mobile hydrogen storage. This provides significant benefits in addressing the energy challenges on the path to net-zero CO2 emissions. The incorporation of hydrogen atoms into the metal lattice of suitable elements and alloys results in a high volumetric storage density (150 kg H2/m3 in Mg2FeH6), which can even exceed that of cryogenically liquefied hydrogen (≈ 71 kg H2/m3 at -253 °C) or hydrogen compressed to 700 bar (≤ 40 kg H2/m3 at 700 bar).
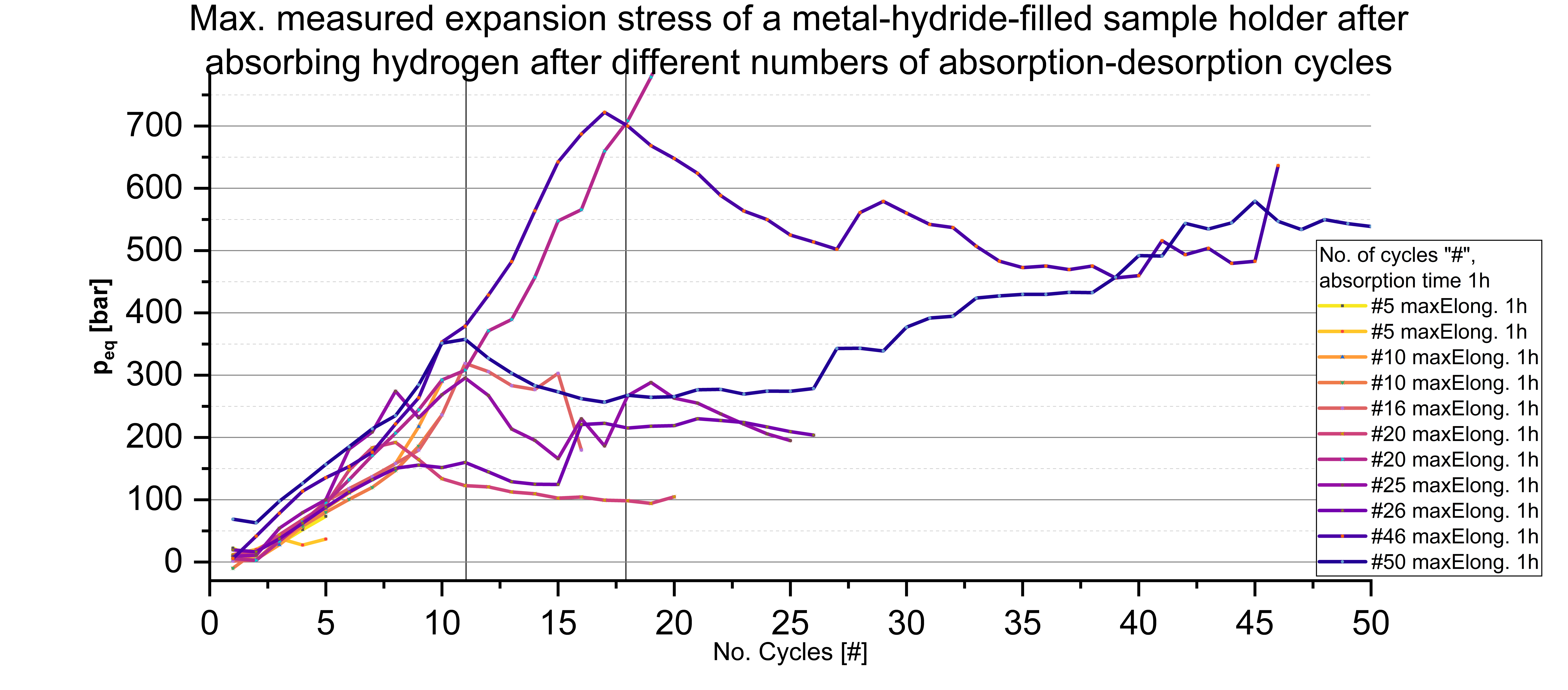
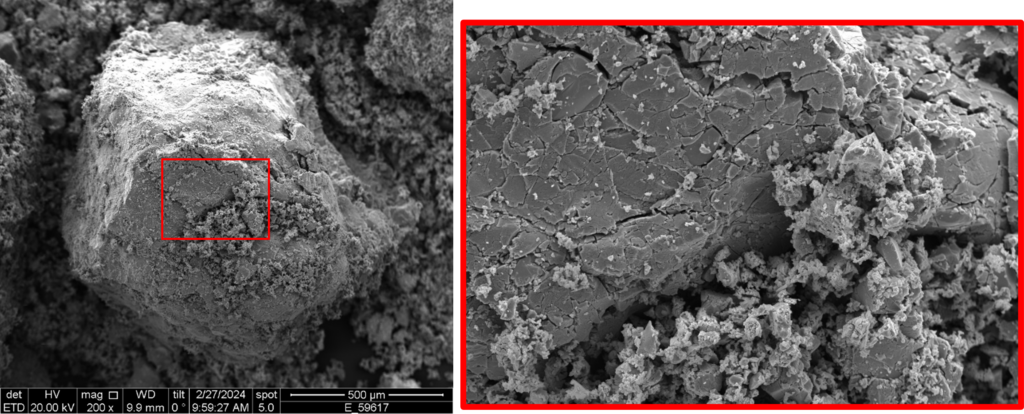
The incorporation of hydrogen atoms into the host lattice causes a powerful expansion of the crystals by up to 30%, which must be managed in the development of storage systems. If not considered, the expansion forces can deform the container wall of the storage system and lead to the development of leakages.
This research field aims to delve deeper into the influencing factors of the expansion forces using state-of-the-art technologies at both the micro and macro levels, and to relate these factors in their complex interrelationships. From this understanding, measures are derived and experimentally tested to reduce the expansion forces while simultaneously making the overall system more efficient by increasing capacities. With these insights, optimized systems with increased hydrogen capacity under controlled storage conditions can be developed, advancing our path towards the energy transition.
Project Partner:
Contact:
Letzte Änderung: 13. October 2025

